What is a physical change? Is there a physical change when you shred a piece of paper? Is there one if you burn a piece of paper? If ice cream that melts from a solid to a liquid or water boils from a liquid to a gas—are those physical changes?
ln the eighth-grade classes of William Bander, Middle School Science Teacher, students learned all about physical changes during an interesting lab.
“Physical changes occur when matter changes in some way (usually in appearance), but no new substances are formed from the change,” explains Bander. “For example, when you rip up a sheet of paper, the pieces are still paper. They still look like paper, feel like paper, smell like paper; you can even write on them just like a full sheet of paper. This is an example of physical change. However, burning paper is not a physical change. The ashes and smoke no longer look, feel, or smell like paper…and you certainly can’t write on ashes or smoke.”
To illustrate this further, students observed three main types of physical changes in the lab. “The three types of physical changes in this lab were shape, mixture, and state,” said Emma Gavin ’30.
First, students crushed up a sugar cube. They placed the cube in a small Ziploc bag and then used their hand to crush it thoroughly. What was the physical change? The shape of the sugar cube changed.
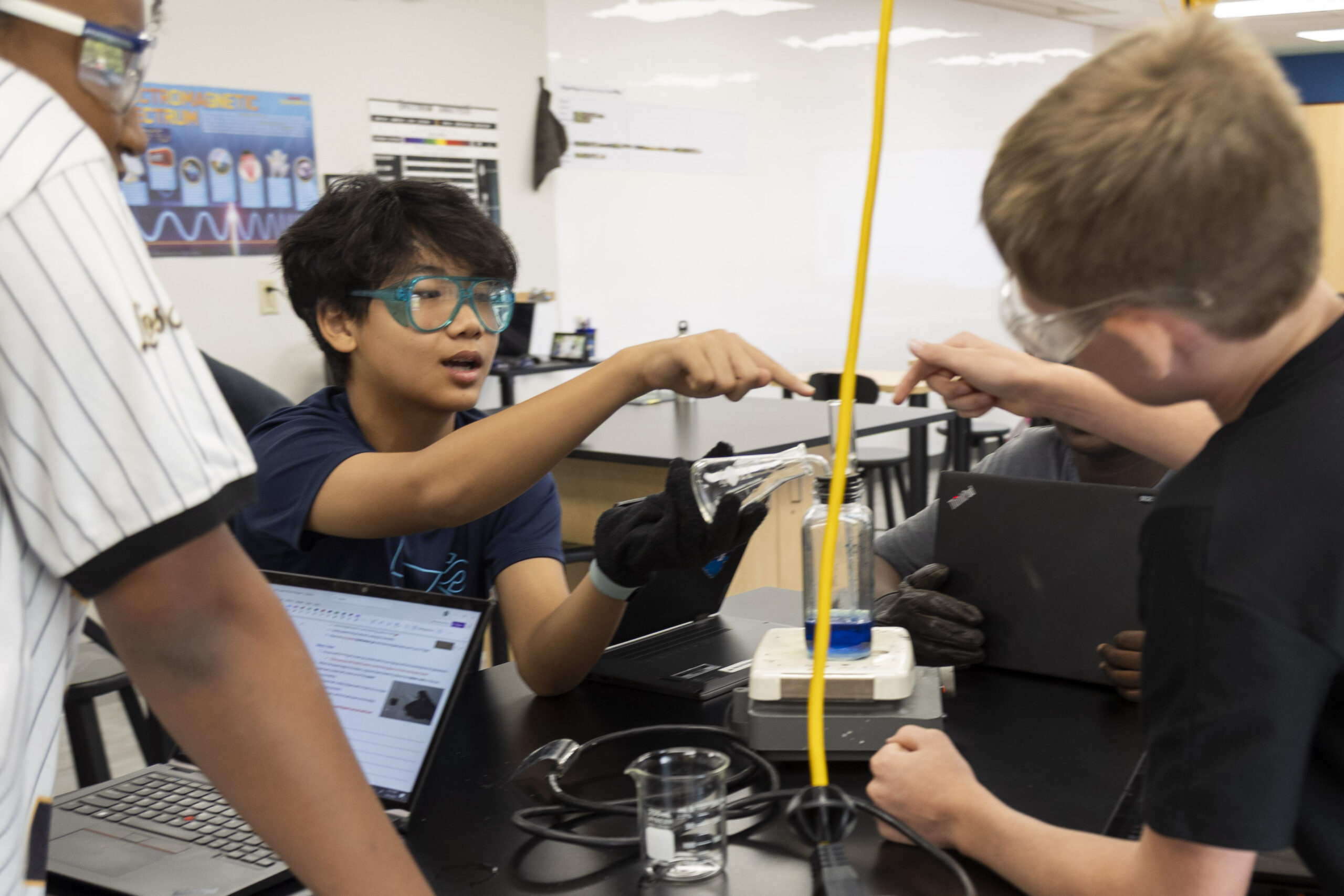
Next, students poured the crushed sugar crystals into a distillation jar. Using a beaker, they added 150 mL of water to the jar plus a single droplet of food coloring. By adding the sugar and food coloring to water, they were mixing substances together. “Adding some food coloring into the water and the act of mixing in the color was a form of physical change,” Gavin said.
Once the food coloring was gently mixed throughout the colored sugary water, the uncorked distillation jar was placed on a hotplate set to “high.” After a few minutes, the colored liquid began to bubble. With gloves on, students placed rubber corks on the jars, which were also equipped with small spouts. Soon, steam evaporated out of the spout like a tea kettle. Students then used a flask to collect steam from the spout for a couple of minutes before setting the flask down on the table and turning off the hotplate.
“By boiling the colored solution, students changed the state of the water from a liquid to a gas,” notes Bander. “What’s really cool is when the students collect the steam in a flask to see that it is clear water, not colored. They can then see that steam is just water in the gaseous state, not a new substance like smoke. Students also deduced that the water collected in the flask will not have any sugar, since only the water evaporated out of the solution, leaving the sugar behind.”
Students really enjoyed this lab. “The lab was very interesting to do,” Gavin said. “I really enjoyed watching the steam collect but also getting to understand how it worked in such a fun way.”
“This lab visually showed us how all these physical changes appear, which really helped me further my understanding,” said Evelyn Holtz ’30. “My favorite example of physical change in this lab was when the water turned into steam. The whole process was a fun way to experiment and learn.”
Izzie Martin ’30 reflected, “The lab helped us understand our unit of chemistry better by showing us how matter can change their physical shape but still remain the same thing. It improved our understanding of matter and how chemistry holds so much importance in our everyday lives. During the lab, it was so fun watching the steam come out of the distillation jar, and I was shocked when our previous purple-dyed mixture went back to its earlier clear color. I then learned that when steam is produced, it’s only water, and it will not carry anything else it has been previously mixed with.”
By the end of the lab, students understood that a physical change alters the state of matter but does not change it into a new substance. In other words, the matter changes, but it still has the same chemical identity. They spouted off other examples, like chopping wood, molding a shape out of Play-Doh, or a penny being flattened by a train on train tracks. Now, as our eighth graders go about their everyday lives, they’ll be even more than ready to notice physical changes in the world thanks to Mr. Bander’s lab!