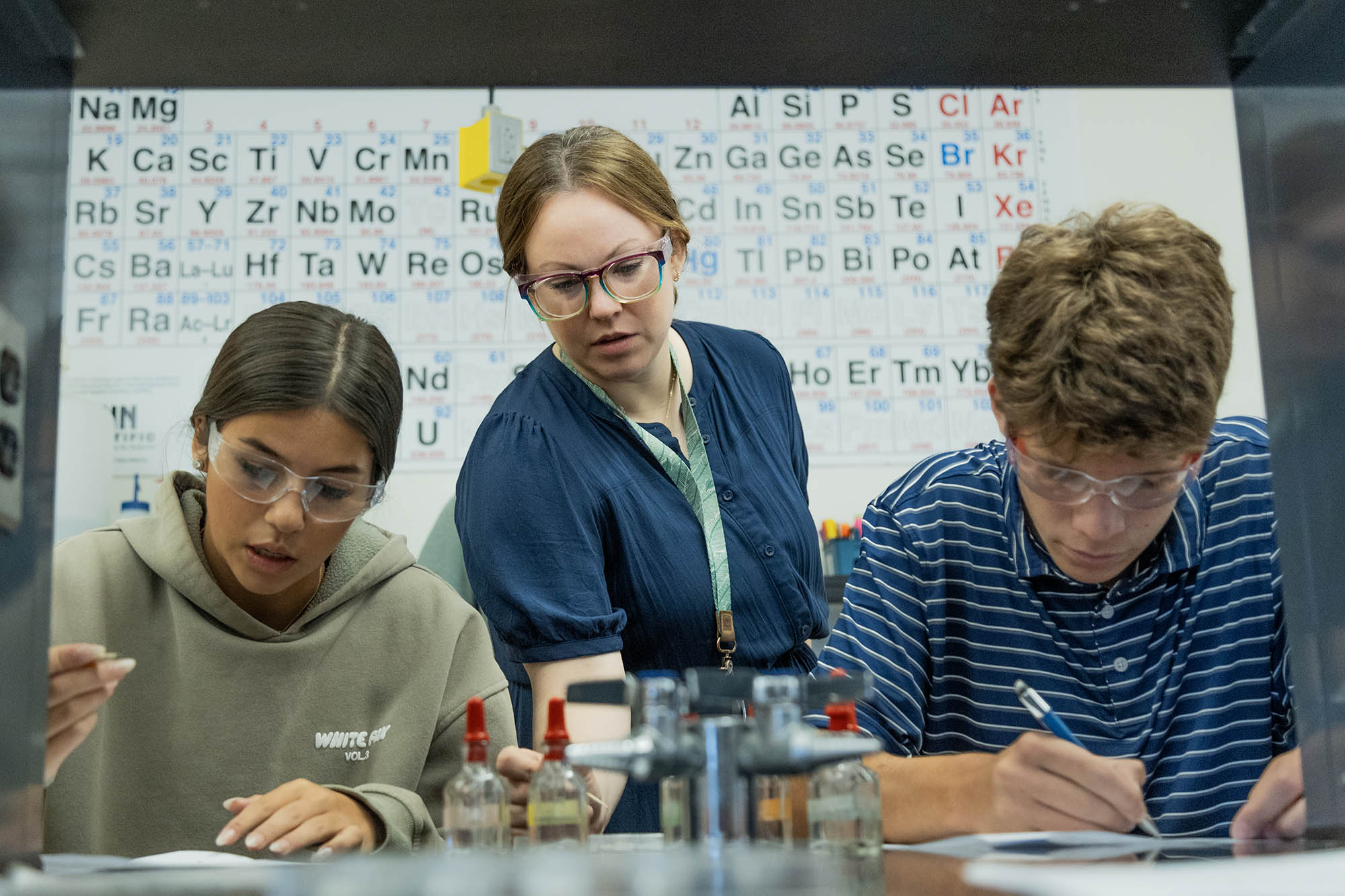What makes a science experiment become an exciting sleuthing experience? Using clues to predict what will happen next! Our students did just that in a recent lab, using a powerful and universal tool: the Periodic Table.
In the Upper School Integrated Chemistry classes of Laura Bradford, Stephanie Matteson, and Dr. Megumi Yoshioka-Tarver, students took a deep dive to understand why salts of certain elements are more soluble than others and how they produce or don’t produce a precipitate solution.
Did you know that a salt is simply a compound made from a metal and a nonmetal? In its most basic form, it is a positive piece and a negative piece coming together to form a whole new substance. For example, ordinary table salt is made of sodium (a metal) and chlorine (a nonmetal).
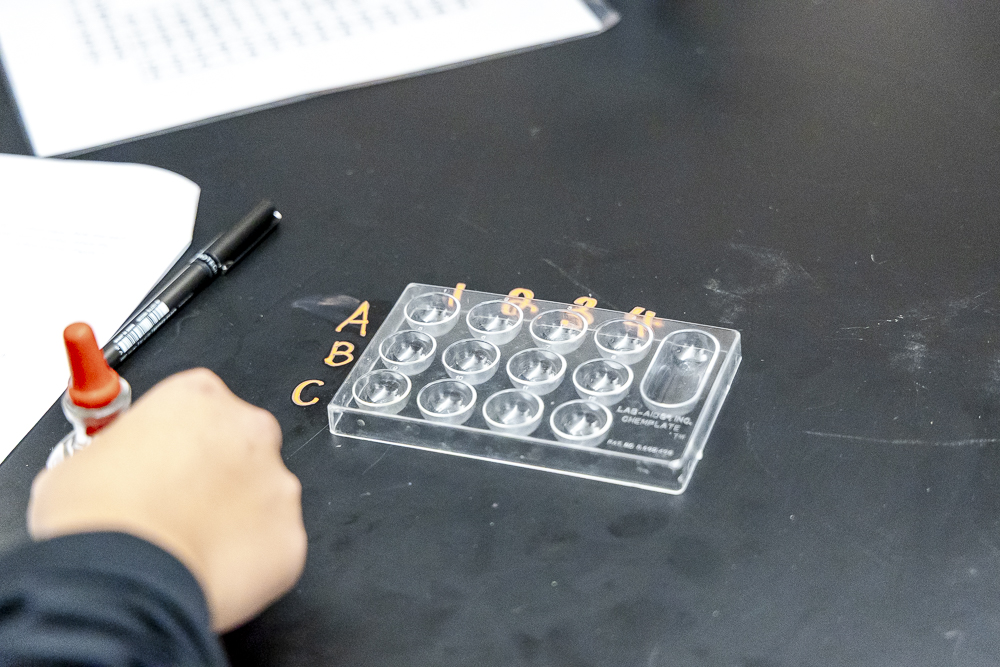
Focusing solely on the salts of the alkaline earth metals (Group 2 of the Periodic Table), students began working on their solubility tests. Their aim? To understand why salts of certain elements are more soluble than others and how they produce or don’t produce a precipitate solution. They used a special tool called a 12-well reaction plate. This mini-lab allowed students to mix different solutions in tiny amounts and carefully observe the results.
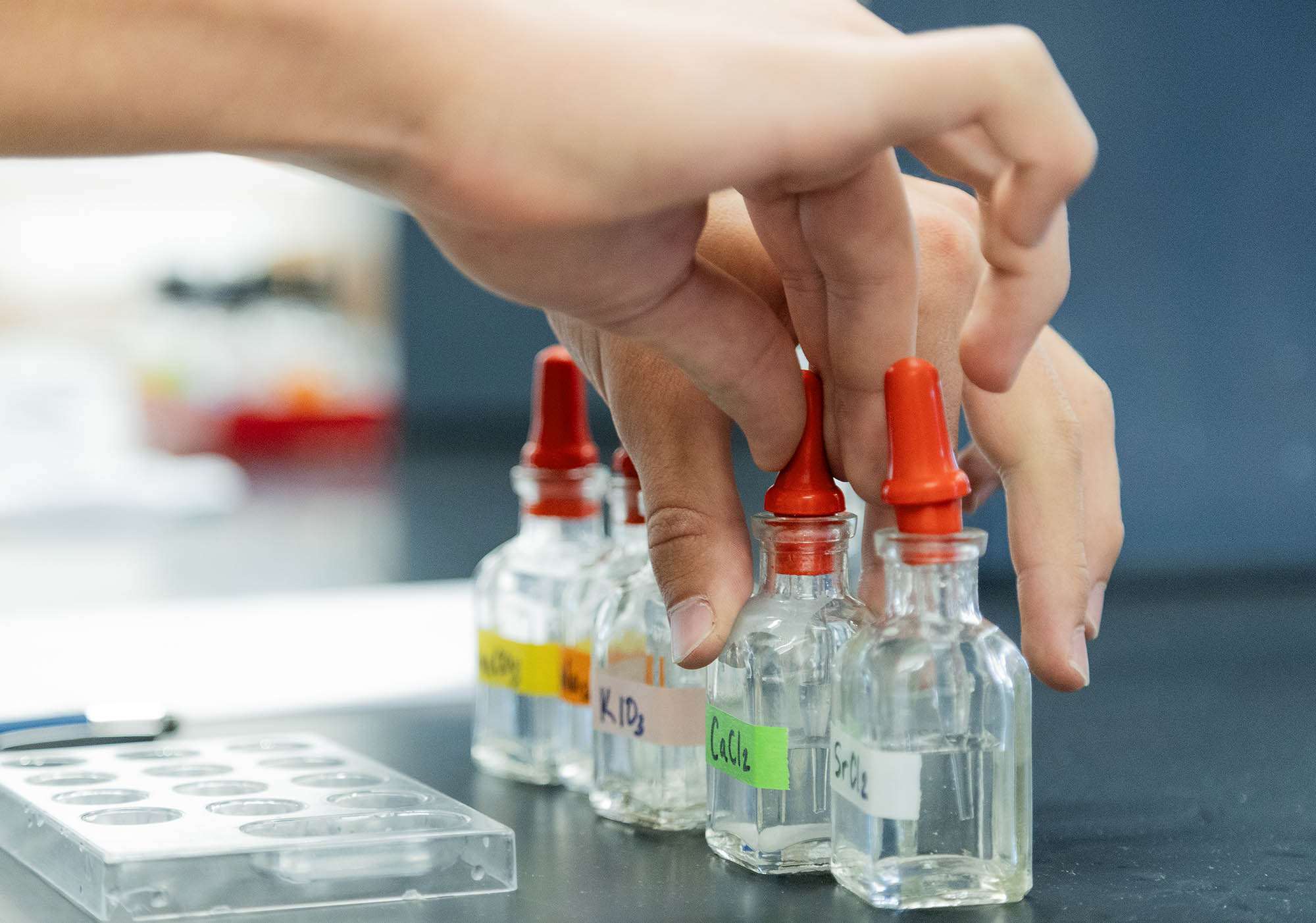
Students added drops of the metal chloride solutions (calcium chloride, magnesium chloride, and strontium chloride) to specific wells, followed by drops of the testing solutions (sodium carbonate, sodium sulfate, and potassium iodate), with observation being the primary goal. Did a precipitate (a cloudy solid) form when the solutions were mixed? If so, the salt is insoluble. If not, the salt is soluble.
When interpreting their collected data, students ranked the species from most to least soluble based on their observations. Then, they developed models to determine if a trend in solubility exists for the salts of the Group 2 metals. To take it a step further, students then used the Periodic Table as a model to predict reactivity trends for alkali metals (Group 1 of the Periodic Table), explaining why sodium is more reactive than lithium.
Audrey Walker ’28 enjoyed facilitating the precipitate lab. “I enjoyed seeing the reactions from different chemicals and how they relate to trends on the periodic table,” she said. “I learned that as you go down group one on the periodic table, the metals become less soluble. I also learned that sodium (Na) is more reactive than lithium (Li) because sodium’s valence electrons are farther away from the nucleus than lithium’s valence electrons, making it easier for sodium’s valence electrons to be pulled away and react with a different chemical.” Kennedy Newman ’28 agreed that witnessing the different reactions was fun. “I enjoyed seeing the different reactions that took place. It was interesting to see which compounds reacted in the liquid and which didn’t.”
Labs like this foster critical thinking and collaboration skills in students, preparing them for advanced scientific studies and equipping them with the adaptability needed to navigate a rapidly evolving scientific landscape.